In brief
On 3 June 2024, the Regulations on hazardous substances for domestic, industrial and/or public health use (Supreme Decree No. 031-2023-SA) (“Regulations“) became effective.
What is the purpose of the Regulations?
The purpose of the Regulations is to regulate and supervise the sanitary control of hazardous substances for domestic, industrial and/or public health use established in Chapter VI of Title II of Law No. 26842, General Health Law.
The Regulations will be enforced by the General Directorate of Environmental Health and Food Safety (DIGESA) of the Ministry of Health.
To whom do they apply?
The Regulations apply to natural and legal persons that carry out activities related to the importation, manufacture, formulation, processing, assembly, distribution, commercialization, storage, use, handling and destruction of hazardous substances for domestic, industrial and/or public health use.
The Regulations include the following provisions:
I. New classification of products in the manufacture of which hazardous substances are used
Formulated products whose manufacture includes the use of hazardous substances for domestic, industrial and/or public health use are classified as follows:
- Chemical products for industrial use
- Inert surface disinfectant products for domestic, industrial and/or public health use, and disinfectant products for swimming pool water
- Pesticide products for domestic, industrial and/or public health use
- Hazardous products under the Rotterdam Convention
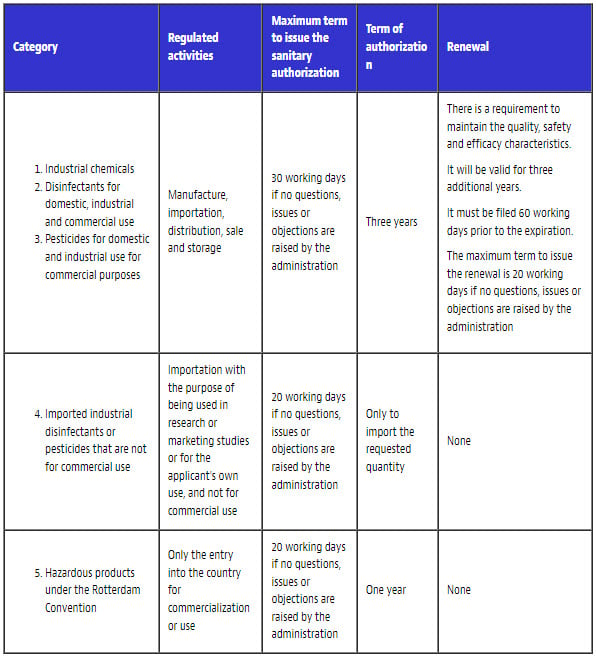
Each product classification has its own requirements and administrative procedures for the respective sanitary authorization to be issued.
II. General aspects of the sanitary authorization
These products require prior authorization from the state (in this case, through DIGESA) to be marketed and used safely without affecting people’s health or the environment.
The most important elements of the procedure for the corresponding authorizations to be issued are the following:
III. Product packaging or labeling
The packaging must contain the product information in accordance with the nationally adopted version of the Globally Harmonized System of Classification and Labeling of Chemicals (GHS).
Regarding the labeling of these products, their primary or secondary packaging must contain legible information in accordance with the GHS and in Spanish. Finally, the Regulations state the minimum information that these products must contain.
The name of the products must not cause confusion about their composition, properties or indications.
IV. Extension and/or modification of sanitary authorizations
The Regulations allow, under the same quality and safety conditions, (i) the modification of fragrances and/or colorants of the authorized products, and (ii) the extension of the product’s scope with respect to other microorganisms or pests different from those authorized for the following products:
- National or imported disinfectants for domestic, industrial and/or public health use for commercial purposes
- National or imported pesticides for domestic, industrial and/or public health use for commercial purposes
V. Revocation, cancellation of the sanitary authorization and stockout
a. Revocation: The sanitary authorization will be revoked when it is determined that the hazardous substances for domestic, industrial and/or public health use are unsafe or ineffective according to the terms under which they were authorized, or that they may affect the health of the population, based on information issued by the WHO, regulatory authorities of other countries or control and sanitary surveillance actions.
b. Cancellation: The holder of the sanitary authorization may request the cancellation of the authorization, being responsible for the collection of the marketed product and its use after cancellation.
c. Stockout: It can be applied when the holder of the sanitary authorization makes modifications or when the validity of the sanitary authorization has expired and provided that the product has no observations regarding its quality or sanitary safety and there are still products in the market.
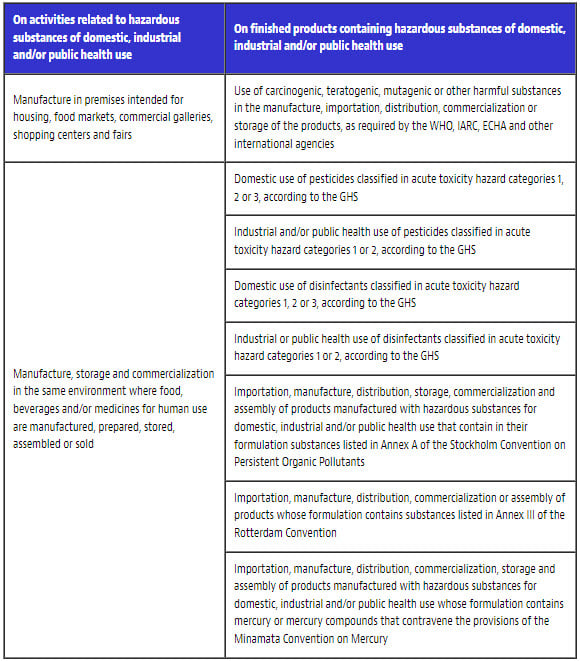
VI. Prohibitions
VII. Control of these products
DIGESA supervises establishments intending to import, manufacture, formulate, process, assemble, commercialize, distribute and store hazardous substances for domestic, industrial and/or public health use, to verify that they comply with the requirements established in the Regulations.
Likewise, DIGESA may take samples from the aforementioned establishments to verify that they comply with the technical specifications stated to obtain the sanitary authorization.
VIII. Violations and penalties
DIGESA is the authority in charge of exercising the sanctioning power with nationwide jurisdiction.
Violations are provided in the Regulations and refer to the noncompliance with prohibitions, obligations and labeling. Those who incur in the infractions typified in the Regulations will be subject to the following penalties:
- Warning
- Temporary or definitive closing of the establishment
- Cancellation of the license
- Fines between 0.5 and 100 tax units
We hope this information is of relevance to you and your company. Please do not hesitate to contact us if you require any advice in this regard.
* * * * *

Estudio Echecopar is a member firm of Baker & McKenzie International, a Swiss Verein with member law firms around the world. In accordance with the common terminology used in professional service organizations, reference to a “partner” means a person who is a partner or equivalent in such a law firm. Similarly, reference to an “office” means an office of any such law firm.
Before you send an e-mail to Estudio Echecopar, please be aware that your communications with us through this message will not create a lawyer-client relationship with us. Do not send us any information that you or anyone else considers to be confidential or secret unless we have first agreed to be your lawyer in the matter. Any information you send us before we agree to be your lawyers cannot be protected from disclosure.
@2024 Estudio Echecopar
All rights reserved.
No part of this publication may be reproduced in any form or by any means without the written permission of Estudio Echecopar.






